Although the use of coffee as a beverage exists noticeably since the thirteenth century, it’s use as a film developer is very recent. Actually, the first reference to successful experiences date back to 1995 through Dr. Scott Williams and his technical photography class in Rochester Institute of Technology (R.I.T. – www.rit.edu/science/williams-research-group ) Their idea was to create a film developer with simple components. Caffeine beverages seemed to be the most efficient substances and coffee was specially effective, so it was chosen to continue the investigation. During their research they found that it wasn’t caffeine that was acting as a developer, but it’s phenolic family of compounds (3). Anyway, their discovery
became immediately popular among the photographers community. The developer got the name “caffenol” in 2000. The idea to use ascorbic acid (vitamin-c) as an additional component for caffenol developer came later and it’s usually referred as caffenol-c (4). Nowadays if we type the word “caffenol” in a internet browser we will easily find dozens of different blogs, websites, photos and even books about it. In this research i started using the coffee based developer as the first step to begin testing a wide range of low-toxic ingredients that could be eligible to use in film emulsion developing. Alternative practices: Coffee, spearmint, hyacinth, hydrogen peroxide, limes and vinegar. Conventional black and white reversal film developing, both in super8, 16mm or still photography (slide film), normally needs highly toxic chemistry in all steps. This happens mainly in developer and bleach. Toxic chemistry include metol, hydroquinone, potassium thiocyanate, potassium
dichromate and other reagents. Some of them are highly toxic, carcinogenic and teratogenic (5-7). For many years, potassium dichromate was used in commercial bleaches, from Kodak to Ilford and other photo-chemistry manufacturers. During the last decade of the twentieth century this
companies changed their potassium dichromate based bleaches after recognizing it’s high toxicity. Potassium permanganate was the most common choice for it’s substitute. But still, potassium permanganate was also toxic, so it didn’t seem to have interest for this particular research. So I also began recognizing that all this known issues about the high toxicity of photographic chemical components seemed to pose a big barrier to open up this practices to many artists, students, and specially children. This also posed a problem to countries were complex chemistry can´t easily be found. In some cases, their high prices are also prohibitive. Finding an alternative low-toxic film reversal process seemed to be an opportunity to open new possibilities and accessibility. Black and white reversal film developing process One of the main laboratory research subjects of this article relates to the use of a low-toxic developer. But for using it with black and white reversal film emulsion there was a need for a safer bleach. Reversal film means that it is designed to be exposed and developed directly to positive image, so it can be projected without the need of a copy from the original negative. This type of film was extremely popular in small cinema super 8 and 16mm cinema formats, and 35mm still slides. Reversal process is normally obtained by a process of six steps, containing first developer, bleach, clearing bath, re-exposure to light, second developer and fixer. The first step (first developer) develops the exposed light-sensitive silver halide crystals to metallic silver (a negative image). Between every step the film must be washed so there is no contamination to the next step. In the second step, the bleach dissolves the metallic-silver negative image produced in the first developer but does not affect the remaining silver halide. The third step removes any bleach left from the wash step and prepares the film for redevelopment (In low toxic reversal processes I decided to skip this step, just rinsing the film with water, since it’s easier to remove hydrogen peroxide from the emulsion). The fourth step is the re-exposure of the film to light, exposing the silver halide crystals that were not exposed in the camera. The fifth step (second developer) develops remaining exposed silver halide to produce a positive image. The last step is fixing, which removes any undeveloped silver halide grains, after the film is dry it’s ready to project. The film used in this research was black and white reversal film Fomapan R100, but Kodak 7266 TRI-X and Kodak High Contrast 7238 film were also tested with excellent results. Film developers are build up of four basic components: one or two developing agents; a preservative, which slows the rate of developer oxidation; an accelerator, which energizes the developer and a restrainer, which restricts the formation of excessive fog and/or slows the rate of development. All four of these components are important for the development process to be successful. However, one chemical component may serve more than one function (as an example, ascorbic acid seems to work as a preservative, as well as a developing agent, this fact is very useful,
since a low toxic component can substitute two toxic components, in this case, hydroquinone and sodium sulfite). This research aimed to substitute the most toxic developing agents first, so that’s why coffee and ascorbic acid (vitamin-c) were commonly used. The preservative was not used (by two reasons, the first was because the objective of this tests was to find the most efficient developer, so, there was no point in keeping the developer for more than a couple of hours, and second because ascorbic acid seemed to act as a preservative as well, so we stopped using sodium sulfite definitely). The most used accelerator was sodium carbonate, it is low toxic, but it can act as an irritant to skin. But since it is minimal, compared with other reagents, it became the most used accelerator (although not so effective, but definitely less irritant, sodium bicarbonate can also be used). The developing agents of the developer used in this research were all rich in phenolic compounds, since they are proved to be very efficient, although the information regarding it is scarce. The first step for finding this agents was to focus on ingredients with high phenolic concentration. A long list showed mainly trees and plants. High phenolic compounds in vegetables are not so easy to detect just by looking at it, spearmint (mentha spicata) and other types of mint seem to be the most effective, but also thyme, vanilla or even bear berry leafs. One interesting fact is that usually, fruits and vegetables that are rich in phenols tend to stain the film in different colors. Spearmint and coffee tend to stain it in different tones of brown, yellow and even red (depending on the dilution, time, temperature, pH value and phenolic extraction method etc.). So, focusing on developing agents, we can say that it is a chemical component that must be able to reduce silver or add an electron to it, and this component can come from many different kinds of substances, organic or not. Hydroquinone is one of the most popular organic developers, but its not so easy to find in nature. Catechol, another developing agent is much more common though, it is present in dozens of plants and fruits. Ascorbic acid is another powerful component, and it is possible to find in many fruits and plants. Some plants or fruits even contain more than one developing agent, bear berry leaves, (uva ursi) contains both hydroquinone, catechol and even a small amount of ascorbic acid.
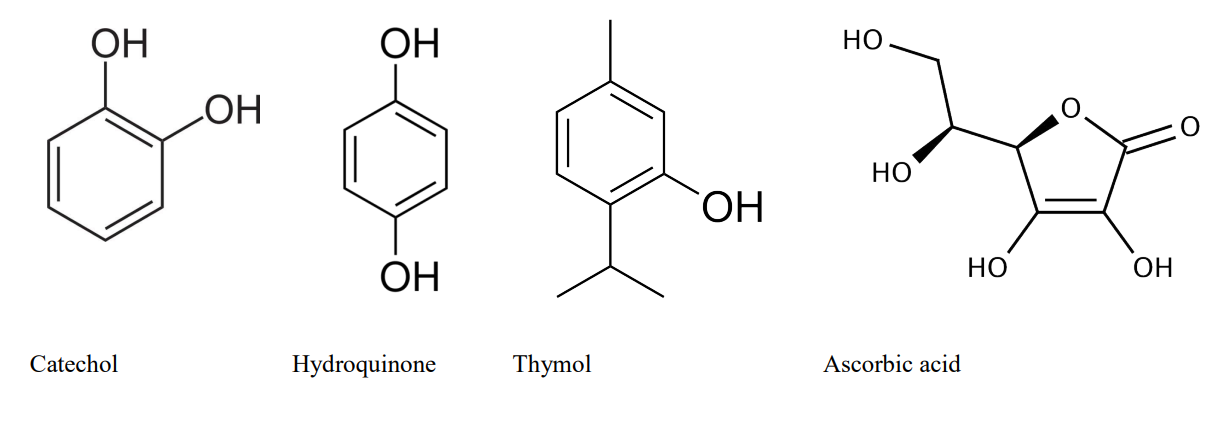
The list can grow even bigger if we consider that its not only possible to reduce silver with plants containing this developing agents, but also others that contain similar molecular structures to this known developing agents. Thyme (thymus vulgaris) is a good example, since it contains thymol, a substance that has a similar molecular structure to the developing agents described earlier. But there are many others, its concentration, method of extraction, availability, cost and other factors can help decide which one is preferable. can is different . Caffenol developer stains the image in a bright brown tone, together with the hydrogen peroxide bleach this tone can vary from a rose-brown to yellow-brown, pH values from both (developer and bleach) can also change the tone. So, changing the developer could also bring more colors to the palette. In a short research period it was clear that
some work had already been done after caffenol regarding new substances . Continuing the research, it was possible to know that some plants were even more rich in phenolic compounds than coffee, and some were cheaper. Spearmint seemed a good choice, it is cheap, easy to grow, rich in phenolic compounds and it was said to have the same effectiveness than coffee. At first, spearmint was boiled for some minutes to extract it’s phenols as it was referred in the patent written by Dianne Iverglynne (8). Further studies suggested more effective methods for extracting the phenolic compounds of the spearmint, adding a solvent, like methanol, and other similar and more complex methods. But to keep the whole process more simple, boiling and filtering became the most used process. Many tests were made with spearmint based developer together with different types of bleaches, changing from hydrogen peroxide with vinegar, acetic acid, or with limes and pure citric acid. Results with reversal film stock were not satisfactory (the oil in spearmint and thyme, seems to reduce the effectiveness of the hydrogen peroxide based bleach, even after washing thoroughly with water, so further tests are still needed, maybe by using a special washing bath). But with negative film a warm oily texture image with a very good detail, gave very interesting results. Spearmint based developer seems like a cheap, clean, and easy to access option (easy to find or plant in northern hemisphere countries) it seems very useful for negative and print film developing, further tests are needed to make it also a good choice for reversal film. Other plants
were tested after spearmint and thyme, providing better results in reversal processing. It’s obvious that a huge list of plants, fruits and seeds can be used to develop film, but this wasn’t the main interest of this research. The objective was to narrow the list to just a couple of developing agents with similar molecular structures that conventional developing agents have. Spearmint for example shows a molecular structure of high similarity with hydroquinone, so it’s mandatory to search only for other ingredients that can show the same or higher degree of molecular similarity.
*Excerpt from the article “SILVER IMAGES, GREEN ALCHEMY
Alternative low toxic and biodegradable film developing processes” written by Ricardo Leite in 2017
References
- Morin, E., “The Cinema, or the Imaginary Man”. Minneapolis: University of Minnesota Press
(2005) 34,41 - Rancière, J., (2000). “The Politics of Aesthetics, The Distribution of the Sensible” Continuum
International Publishing Group, New York - Williams, S., “A Use for that Last Cup of Coffee: Film and Paper Development”,
http://people.rit.edu/andpph/text-coffee.html Accessed in 21 of May 2016 - Reinhold, G., et al.(2012).”The Caffenol Cookbook & Bible”. Community spirits Publications,
USA. - Norseth T. (1981). “The carcinogenicity of chromium.”Environmental Health Perspectives, 40:
121-30. - Kolaciski Z., et al. (1999). “Acute potassium dichromate poisoning: a toxicokinetic case study.”
Journal of Toxicology – Clinical Toxicology, 37(6): 785-91. - Regev L., Wu M., Zlotolow R. and Brautbar N. (2012). “Hydroquinone, a benzene metabolite,
and leukemia: A case report and review of the literature.”Toxicology and Industrial Health, 28: 64. - Iverglynne D., (2002). “A Biodegradable developing solution and method of use.” World
Intellectual Property Organization - Bertucci S. et al. (1997) “Non-rehalogenating bleaching composition and its use to process
silver halide photographic elements” United States Patent, Number: 5,641,616 – 1997 - http://www.h2o2.com/files/PeroxyChem_35_SDS.PDF Accessed in 21 of May 2016
- Heudorf U., Mersch-Sundermann V., Angerer J. (2007). “Pthalates, Toxicology and exposure.”
International Journal of Hygiene and Environmental Health
Deixe um comentário